Fda Guidance Bcs Classification
The US Food and Drug Administration (FDA) on Thursday released its version of the International Council for Harmonisation (ICH) biopharmaceutics classification system (BCS) -based biowaivers guideline for consultation. The draft guideline, which reached Step 2b of the ICH process in June, is currently under consultation by the group’s regulatory members and is expected to be adopted in May 2019.
FDA is asking interested parties to submit their comments on the guideline within 90 days. In August, ICH released a explaining the guideline, which is meant to create a harmonized approach to biopharmaceutics classification of drugs and to provide recommendations for when in vivo bioequivalence (BE) studies can be waived. “ In vivo BE studies are needed to demonstrate lack of impact of significant formulation changes on a drug’s bioavailability during its development, for post-approval line extensions, and when developing a generic product,” FDA writes, adding that the guideline will help avoid unnecessary BE studies in humans.
Bcs Classification Of Drugs
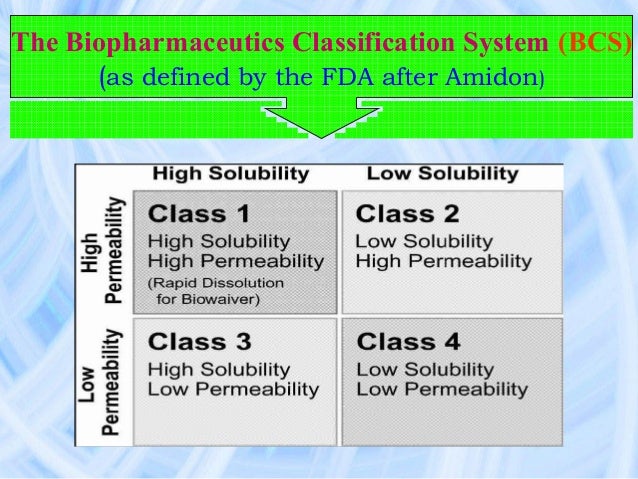
According to ICH, guidelines in the US, EU, Japan, Canada and from the World Health Organization (WHO) provide slightly different recommendations on bioequivalence and biowaivers. Under the biopharmaceutics classification system, drugs are grouped into four classes based on aqueous solubility and intestinal permeability. Biopharmaceutics Classification System (BCS) Class I: high solubility, high permeability Class II: low solubility, high permeability Class III: high solubility, low permeability Class IV: low solubility, low permeability According to the guideline, BCS-based biowaivers only apply to drugs that fall under BCS Class I or Class III and are immediate-release, solid oral drugs or suspensions meant to deliver a drug for systemic circulation. BCS-based biowaivers do not apply to drugs with a narrow therapeutic index and only apply to fixed-dose combination (FDC) products when all the drug substances in the product meet the criteria established by the guideline for individual drugs.
Category Title Type Date Biopharmaceutics Final Guidance 05/21/18 Biopharmaceutics Draft Guidance 04/03/03 Biopharmaceutics Draft Guidance 04/11/03 Biopharmaceutics Final Guidane 06/02/95 Biopharmaceutics Final Guidance 08/01/97 Biopharmaceutics Final Guidance 09/01/97 Biopharmaceutics Final Guidance 12/01/02 Biopharmaceutics Final Guidance 06/27/89 Biopharmaceutics Final Guidance 02/01/01 Biopharmaceutics Final Guidance 12/22/17 Biopharmaceutics Draft Guidance 12/04/13 Biopharmaceutics Draft Guidance 03/17/14 Biopharmaceuticals Final Guidance 08/08/18.
